Since February 2020, the coronavirus has been affecting our everyday life, a major challenge for all of us. R-Biopharm continuously faces its social responsibility to meet the diagnostic requirements for the detection of SARS-CoV-2.
With more than 30 years’ experience in clinical diagnostics, we already support the healthcare sector in containment and control of the coronavirus pandemic with our SARS-CoV-2 PCR assay. R-Biopharm constantly adapts and expands its portfolio to the new requirements of the COVID-19 pandemic.
All RIDA®GENE SARS-CoV-2 tests detect the new variant Omicron (B1.1.529) with the usual sensitivity and reliability.
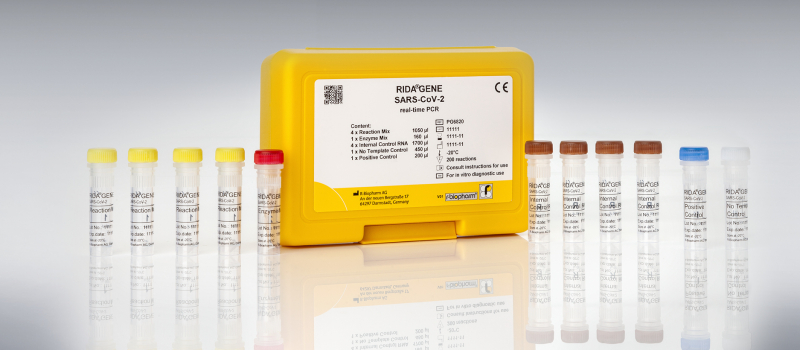
Multiplex real-time RT-PCR for the direct qualitative detection of the coronavirus (SARS-CoV-2) RNA.
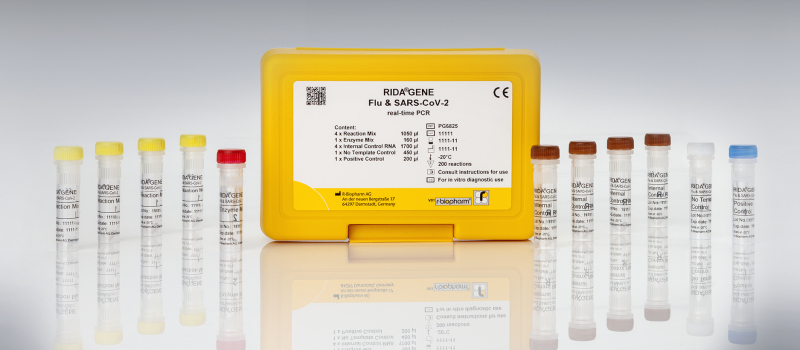
Multiplex real-time RT-PCR for the direct qualitative detection and differentiation of the influenza A/B and coronavirus (SARS-CoV-2) RNA.
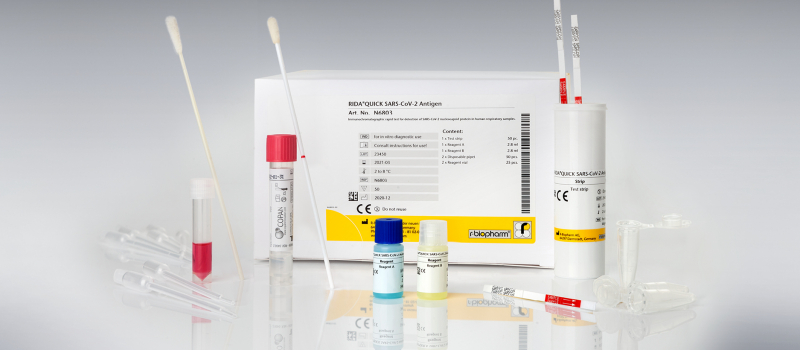
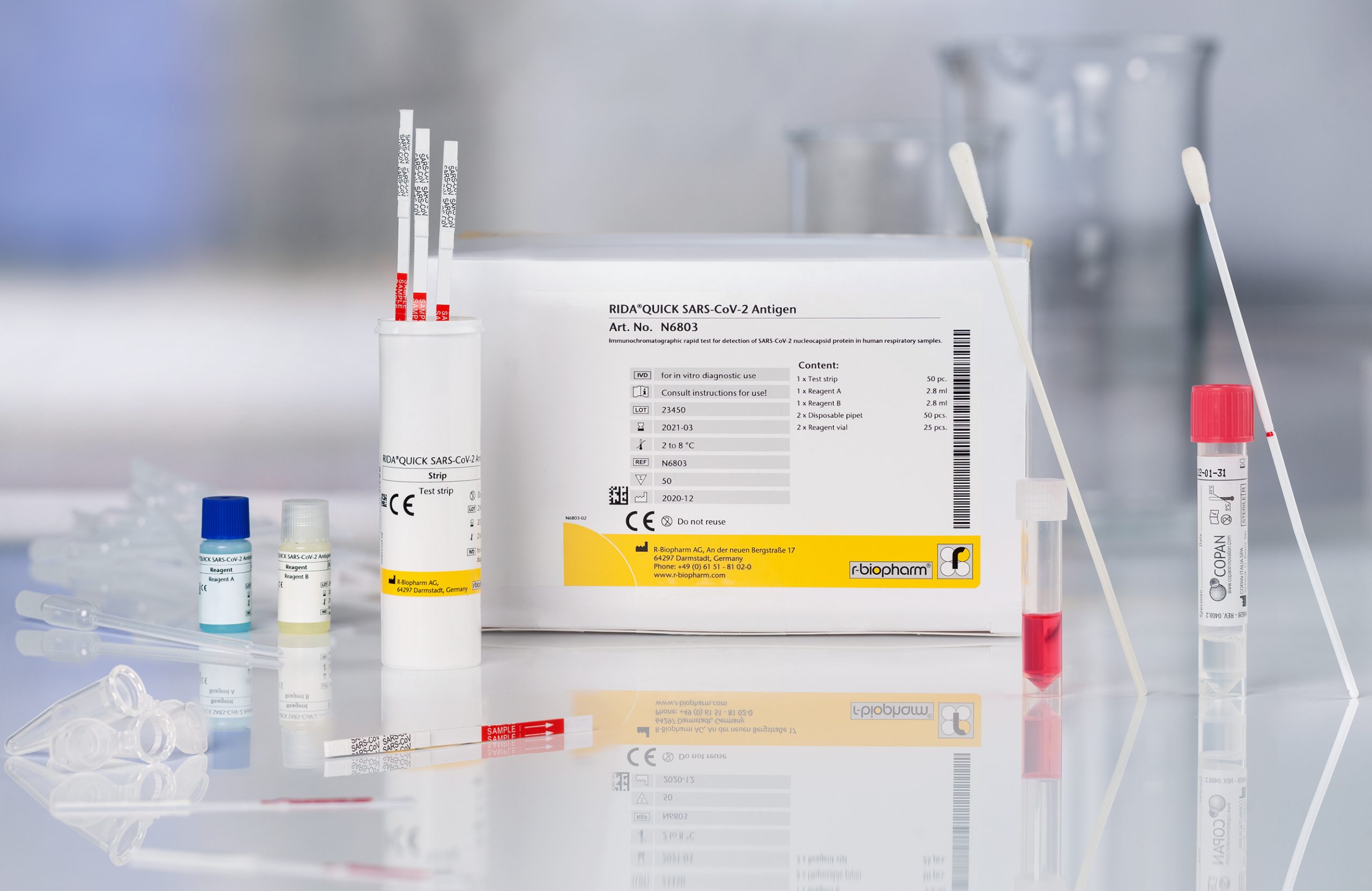
Immunochromatographic rapid test for the qualitative detection of SARS-CoV-2 specific antigens.
RIDA®QUICK SARS-CoV-2 antigen rapid test
- Official partner of the German Federal Ministry of Health for SARS-CoV-2 diagnostics to fight COVID-19
- Easy test procedure including a swab sampling collection device
- Swab system with duplex- transport media allows for PCR-confirmation testing from the same sample applicable for nasal and / or throat swabs
- BfArM (Federal Institute for Drugs and Medical Devices) listed antigen test for the direct detection of the coronavirus SARS-CoV-2
- According to Paul-Ehrlich-Institute, the analytical sensitivity corresponds to the state of the art
- Sensitivity of 95 % with a PCR-Ct value of < 29, as well as a specificity of 100 % (study by the Charité, Prof. Dr. Drosten and Frankfurt University Hospital, Prof. Dr. Ciesek)
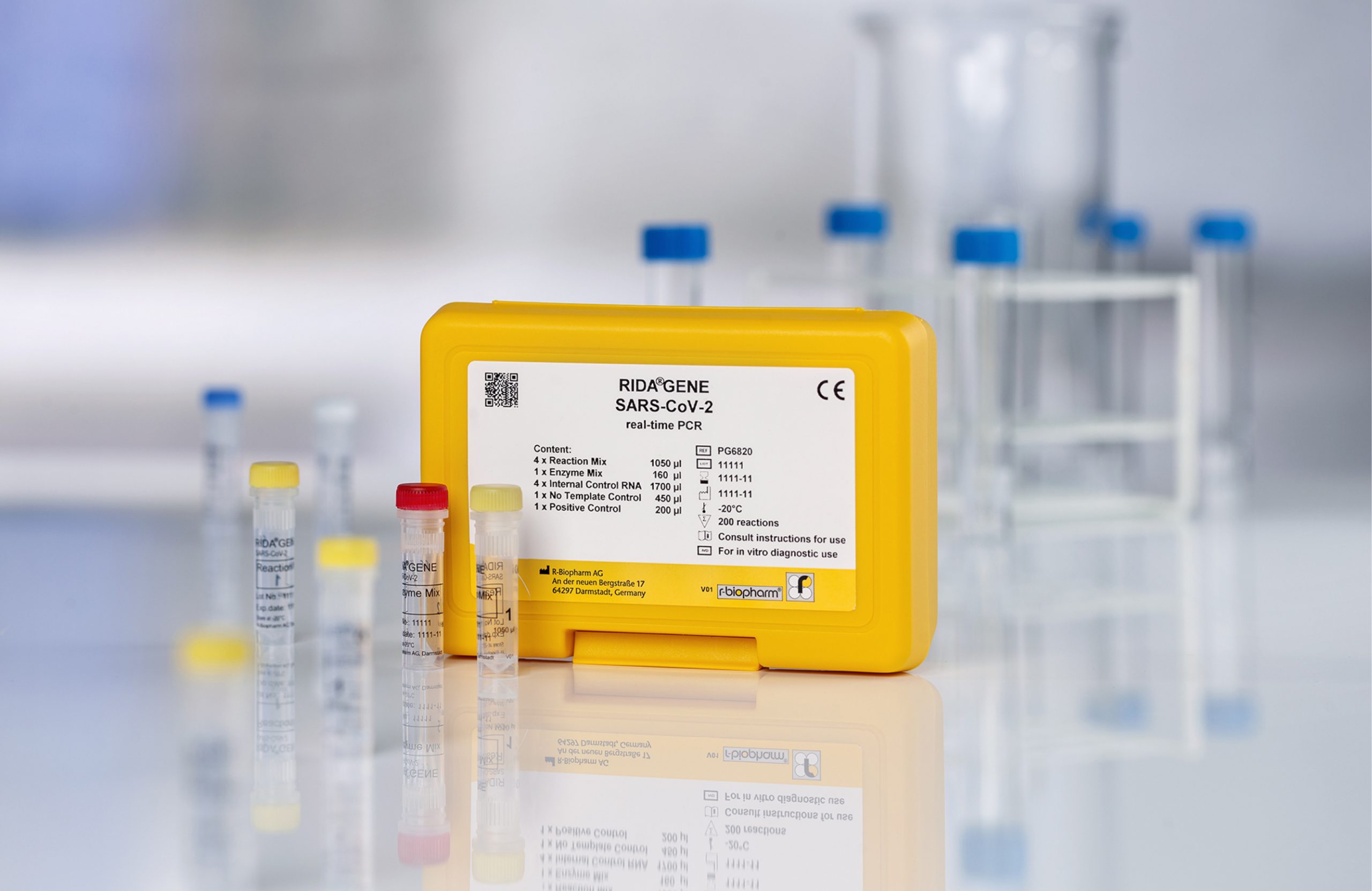
RIDA®GENE SARS-CoV-2 real-time PCR test
- Qualitative specific detection of SARS-CoV-2 (E gene) from respiratory sample material
- The assay can be run on commonly used real-time PCR instruments
- Results in less than 1.5 h
- FindDx (Foundation for Innovative New Diagnostics) listed PCR test
- Suitable for laboratories with high throughput for the initial diagnosis and as confirmatory test after a positive rapid test result
- Meets the WHO recommendation for areas where COVID-19 virus is widely spread
- All controls (internal, positive, negative) are included in the kit
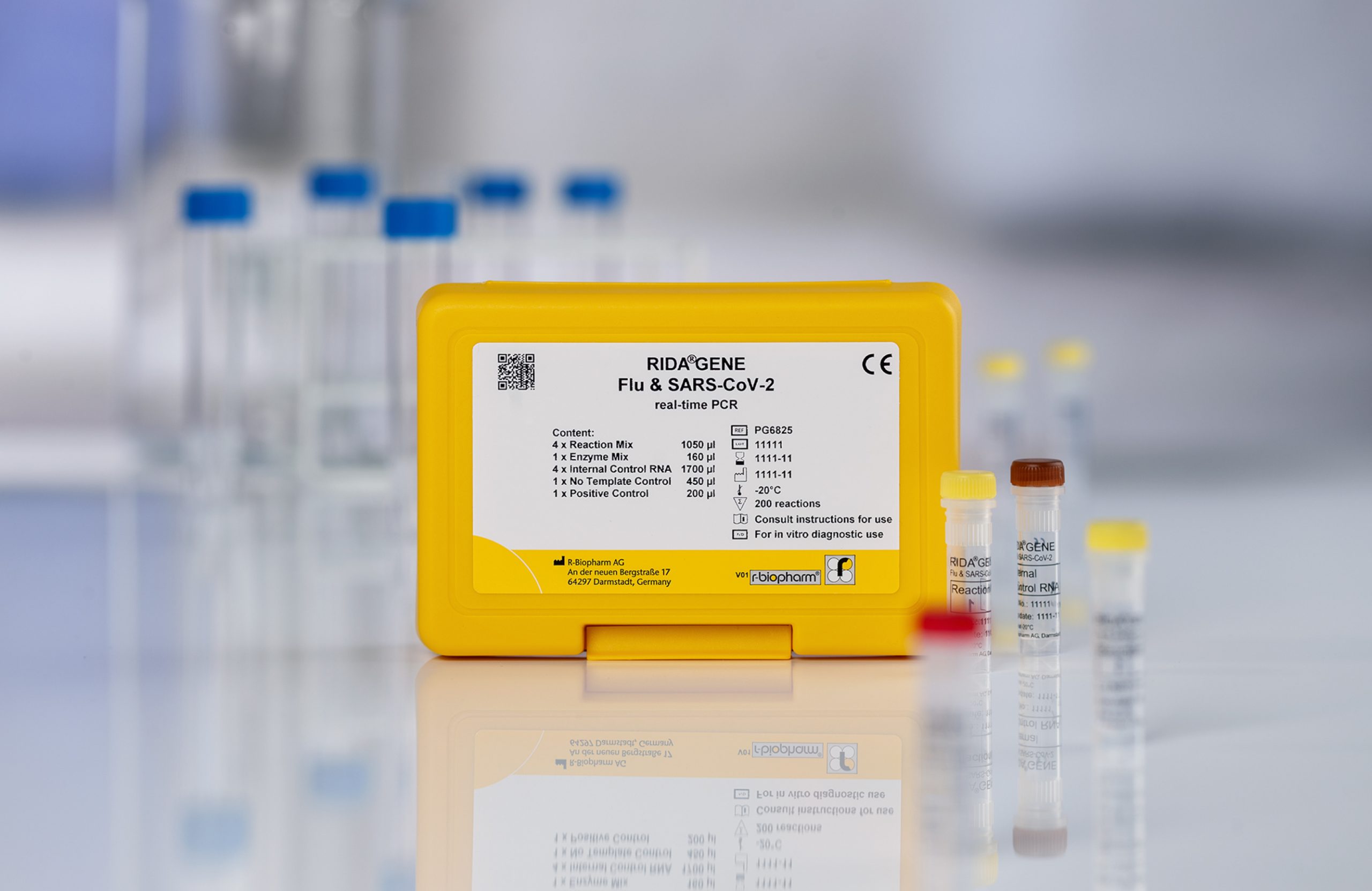
RIDA®GENE Flu & SARS-CoV-2 multiplex test
- Qualitative detection and differentiation of Influenza A/B (M gene/NP1 gene) and SARS-CoV-2 (E gene; RdRp gene) respiratory sample material
- The assay can be run on commonly used real-time PCR instruments
- Results in less than 1.5 h
- Detection of two SARS-CoV-2 specific gene fragments within the E gene and RdRp gene
- All controls (internal, positive, negative) are included in the kit